Scope of Practice and Compensation for Services
Community pharmacists are both medication experts and America's most accessible health care providers. The services they can provide to their patients and communities extend far beyond dispensing, but current laws and regulations can create barriers to those services. NCPA is committed to working with our members and state partners to remove the barriers preventing pharmacists from practicing to the full scope of their training and expertise.
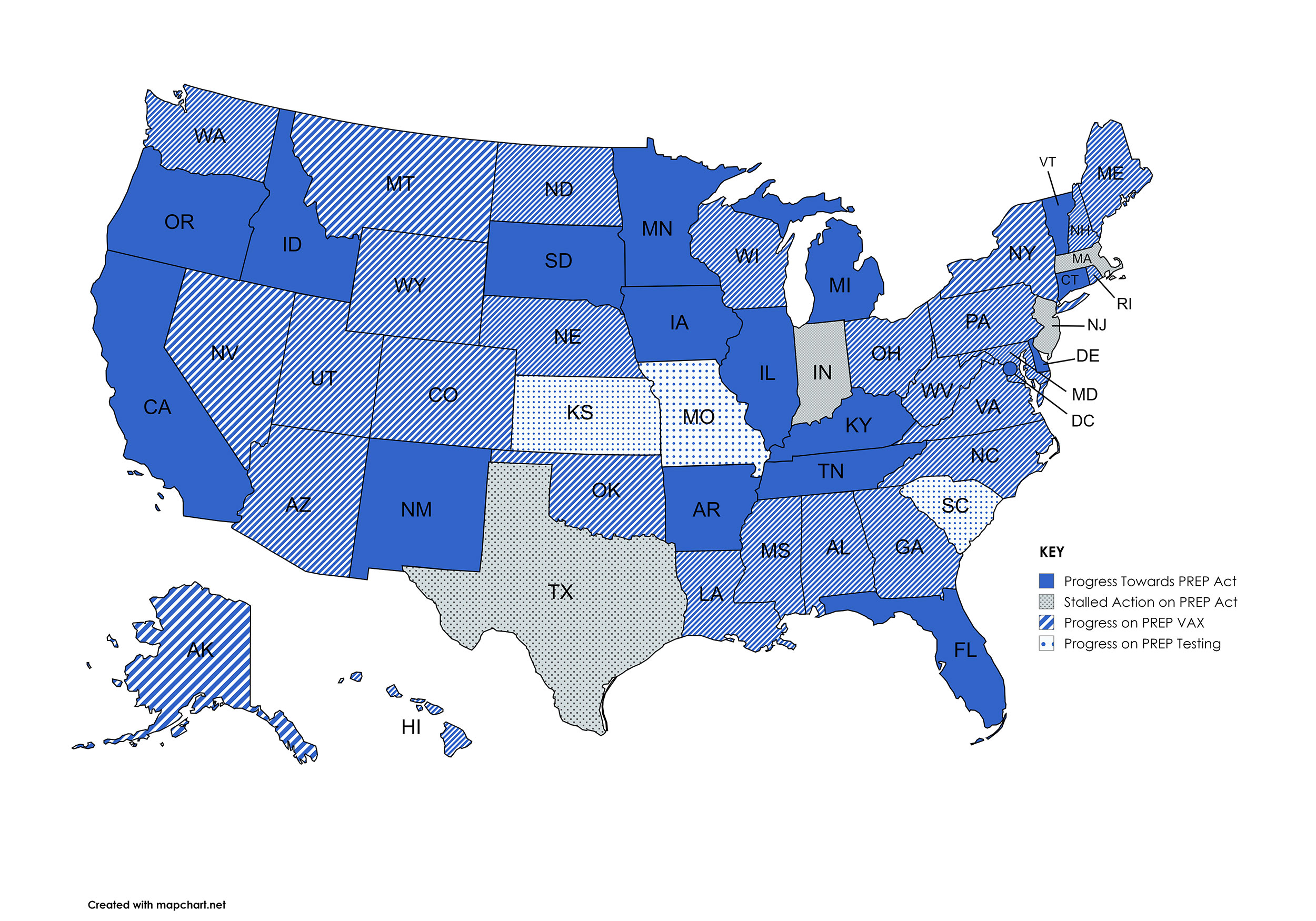
While certainly not a comprehensive list, the following are areas where NCPA is active related to expansion of pharmacists’' scope of practice and insurance coverage or compensation for pharmacists' patient care services.
- Immunizations
- Hormonal Contraceptives
- Tobacco Cessation
- Point-of-Care Testing
- Long Acting Injectables/Medication Administration
PREP Act amendments for pharmacists’ vaccine and testing authority extended through 2029
The U.S. Department of Health and Human Services (HHS) extended the PREP Act protections for pharmacists' vaccine and testing authority through December 31, 2029. The decision allows pharmacists, pharmacy interns, and pharmacy technicians to continue independently administering vaccines and test to treat services for COVID-19. The authority was set to expire at the end of 2024. NCPA was instrumental in advocating this change with HHS. Since 2020, licensed pharmacists in all 50 states have been able to use this federal authority to expand vaccination and testing services to more patients where it hadn’t previously been permitted within the state’s pharmacy scope of practice. Details on the extension will be published on the Federal Register here.
Letters of Support and Comments
Practice Authority and Payment for Service
Hawaii
Illinois
Maryland
Massachusetts
Missouri
Rhode Island
-
S 2329 – Tobacco Cessation in Rhode Island (joint letter with APhA and NASPA)
-
S 2330 – Contraception Prescribing Authority in Rhode Island (joint letter with APhA and NASPA
South Carolina
Wisconsin